Our research has helped to understand the redox reactions of important small molecules like N2, CO2, and hydrocarbons. We often use strategies that are inspired by natural enzymes, in particular the ways that inexpensive metals can be used.
In previous times, we developed nickel catalysts for the reduction of protons to hydrogen (H2) through collaboration with Richard Eisenberg and Todd Krauss at the University of Rochester. These experiments used a light-harvesting molecule (“chromophore”), an electron donor, and a catalyst to produce H2. Using photophysical and electrochemical methods, we made several advances in understanding the stability and reactivity of catalysts with cobalt and nickel. Using CdSe quantum dots, we developed the first homogeneous systems that can form H2 for weeks without decomposition.
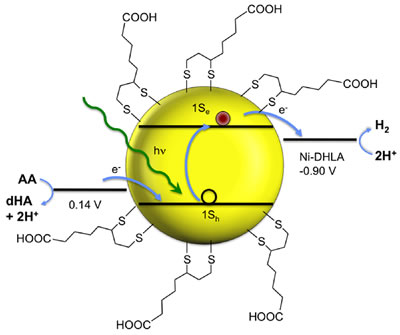
However, in the long run, hydrogen is not a very convenient energy-storing molecule since it is difficult to store and has low energy density. Our current goal is to take advantage of our expertise on N2 reduction to store energy instead in NH3, an exciting frontier in carbon-free energy storage. (See a recent article at this link.) This requires adapting our N2-reducing compounds to perform at milder potentials, with good catalytic turnover, with the electrons coming from an electrode. We have two independent efforts along these lines: one on bimolecular N2 splitting together with Alan Goldman (Rutgers), Faraj Hasanayn (American University Beirut) and Alex Miller (UNC Chapel Hill) supported by funding from the National Science Foundation, and another on tethered complexes for mononuclear N2 activation as part of the DOE-funded CHASE Solar Hub based at UNC Chapel Hill.
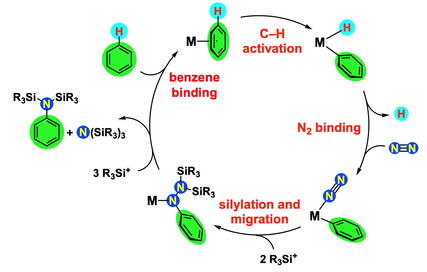
In a separate direction in our lab, we are pursuing catalytic reactions to activate hydrocarbons and couple them with N2 to form N-containing organic compounds (see illustration above). This exciting area combines C-H activation and N-N activation, and forms C-N bonds from abundant resources. This direction in the lab is funded by a grant from the Department of Energy.
