Ryan S. Donnelly, Patrick L. Holland
Alkali Metal Cation Effects on Dinitrogen Complexes and Organometallic Compounds
Acc. Chem. Res. 2026, 59, in press.
|
|
S. McGuigan, S. J. Tereniak, A. Smith, S. Jana, C. L. Donley, L. Collins, N. Ghorai, Y. Xu, E. A. Fosu, S. Suhr, H. R. M. Margavio, H. Yang, G. N. Parsons, P. L. Holland, E. Jakubikova, T. Lian, P. A. Maggard
Illuminating the Mechanistic Impacts of Functionalizing Crystalline Poly(triazine imide) with an Fe Quaterpyridine Molecular Catalyst for Photocatalytic CO2 Reduction
Inorg. Chem. Front. 2025, 12, 6640-6654.
|
|
Dana M. Feldman, Kevinjeorjios Pellumbi, Igor Zimmermann, Wiebke Wiesner, Sebastian A. Sanden, Simon C. B. Suhr, Patrick L. Holland, Ulf-Peter Apfel
Integrating Salen Complexes into Gas Diffusion Electrodes as Precatalysts for CO2 Electroreduction: Considerations for Employing Molecular Electrocatalysts in Heterogeneous Electrolyzers
Chem. Commun. 2025, 61, 16974-16977.
|
|
Alexandre T.-Y. Genoux, Stephen J. Tereniak, Patrick L. Holland
Synthesis of new chelating phosphines containing an aryl chloride group
Synthesis 2023, 55, 2737-2741.
|
|
Alexander S. Hegg, Brandon Q. Mercado, Alexander J. M. Miller, Patrick L. Holland
Catalytic Reduction of Dinitrogen to Ammonia using Molybdenum Porphyrin Complexes
Faraday Disc. 2023, 243, 429-449.
|
|
Gannon P. Connor, Daniel Delony, Jeremy E. Weber, Brandon Q. Mercado, Julia B. Curley, Sven Schneider, James M. Mayer, Patrick L. Holland
Facile conversion of ammonia to a nitride in a rhenium system that cleaves dinitrogen
Chem. Sci. 2022, 13, 4010-4018.
|
|
Gannon P. Connor, Brandon Q. Mercado, Hannah M. C. Lant, James M. Mayer, Patrick L. Holland
Chemical oxidation of a coordinated PNP-pincer ligand forms unexpected Re-nitroxide complexes with reversal of nitride reactivity
Inorg. Chem. 2019, 58, 10791-10801.
|
|
Amit Das, Zhiji Han, William W. Brennessel, Patrick L. Holland, Richard Eisenberg
Nickel Complexes for Robust Light-Driven and Electrocatalytic Hydrogen
Production from Water
ACS Catal. 2015, 5, 1397-1406.
|
|
Zhiji Han, Luxi Shen, William W. Brennessel, Patrick L. Holland, Richard Eisenberg
Nickel Pyridylthiolate Complexes for the Photocatalytic Production of Hydrogen from Aqueous Solutions in Noble-Metal-Free Systems
J. Am. Chem. Soc. 2013, 135, 14659-14669.
|
|
Zuofeng Chen, Christopher R. K. Glasson, Patrick L. Holland, Thomas J. Meyer
Electrogenerated polypyridyl ruthenium hydride and ligand activation for water reduction to hydrogen and acetone to iso-propanol
Phys. Chem. Chem. Phys. 2013, 15, 9503-9507.
|
|
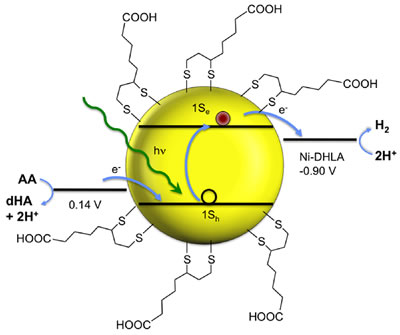
Zhiji Han, Fen Qiu, Richard Eisenberg, Patrick L. Holland, Todd D. Krauss
Robust Photogeneration of H2 in Water Using Semiconductor Nanocrystals and a Nickel Catalyst
Science 2012, 338, 1321 1324.
|
|
Christopher R. K. Glasson, Wenjing Song, Dennis L. Ashford, Aaron Vannucci, Zuofeng Chen, Javier J. Concepcion, Patrick L. Holland, Thomas J. Meyer
Self-Assembled Bilayers on Indium-Tin Oxide (SAB-ITO) Electrodes: A Design for Chromophore-Catalyst Photoanodes
Inorg. Chem. 2012, 51, 8637-8639.
|
|
William R. McNamara, Zhiji Han, Chih-Juo Yin, William W. Brennessel, Patrick L. Holland, Richard Eisenberg
Cobalt-Dithiolene Complexes for the Photocatalytic and Electrocatalytic Reduction of Protons in Aqueous Solutions
Proc. Natl. Acad. Sci. USA 2012, 109, 15594-15599.
|
|
Zhiji Han, William R. McNamara, Min-Sik Eum, Patrick L. Holland, Richard Eisenberg
A Nickel Thiolate Catalyst for the Long-Lived Photocatalytic Production of Hydrogen in a Noble-Metal-Free System
Angew. Chem. Int. Ed. 2012, 51, 1667-1670.
|
|
Theresa M. McCormick, Zhiji Han, David J. Weinberg, Patrick L. Holland, Richard Eisenberg
The Impact of Ligand Exchange in Hydrogen Production from Cobaloxime-Containing Photocatalytic Systems
Inorg. Chem. 2011, 50, 10660-10666.
|
|
William R. McNamara, Zhiji Han, Paul J. Alperin, William W. Brennessel, Patrick L. Holland, Richard Eisenberg
Cobalt-Dithiolene Complex for the Photocatalytic and Electrocatalytic Reduction of Protons
J. Am. Chem. Soc. 2011, 133, 5123-5132.
|
|
Matthew P. McLaughlin, Theresa M. McCormick, Richard Eisenberg, Patrick L. Holland
A stable molecular nickel catalyst for the homogeneous photogeneration of hydrogen in aqueous solution
Chem. Commun. 2011, 47, 7989-7981.
|
|
Azwana R. Sadique, William W. Brennessel, Patrick L. Holland
A diketiminate-bound diiron complex with a bridging carbonate ligand
Acta Cryst. C 2009, 65, m174-m176.
|
|
Azwana R. Sadique, William W. Brennessel, and Patrick L. Holland
Reduction of CO2 to CO Using Low-Coordinate Iron: Formation of a Four-Coordinate Iron Dicarbonyl Complex and a Bridging Carbonate Complex
Inorg. Chem. 2008, 47, 784-786.
|
|