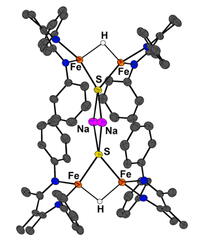
We are motivated to prepare new iron-sulfur clusters, because these are important biological cofactors that can perform amazing reactions like N2 and CO2 reduction, and because they have unusual electronic structures that are a challenge to modern chemistry. In our work, we have prepared numerous unusual clusters and a few of them are shown below.
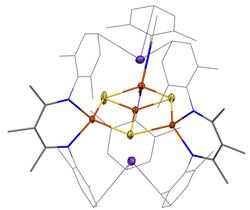
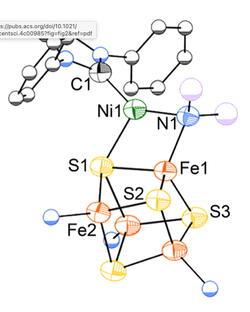
A current topic of research is iron-nickel-sulfur clusters, which are motivated by the active site of CO dehydrogenase, an enzyme that efficiently interconverts CO2 and CO. We are exploring the feasibility of unprecedented metal geometries and oxidation states, with the goal of understanding what is possible in the natural system.