Current efforts by chemists to design sustainable catalysts often focus on replacing heavy metals with Earth-abundant metals that are less harmful to the environment. However, the supporting ligands are another potentially expensive, toxic component of a catalyst. We envision catalysts that are instead composed of non-toxic iron ions, supported by protein scaffolds that are completely biodegradable. This strategy is inspired by enzymes, many of which contain iron; however, natural enzymes have evolved to meet the challenges of survival rather than the broader range of synthetic challenges faced by chemists in the 21st century. Thus, we modify the metalloproteins to encourage new reactivity.
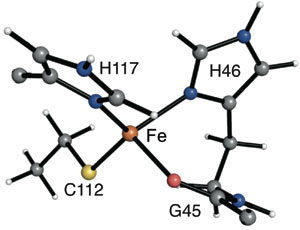
Our first foray into this area used the small protein apo-azurin, which in nature holds a low-coordinate copper center. By substituting iron in the active site and changing the surroundings of the metal site, we were able to bind cyanide and azide. It was also reduced to the unusual iron(I) oxidation state. In future work, we aim to create catalysts with other transition metal-protein assemblies.
